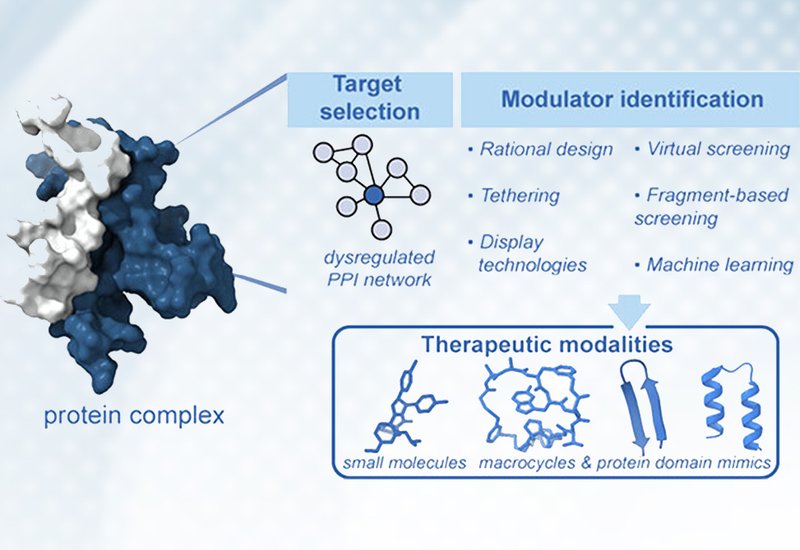
The manuscript titled "From Concepts to Inhibitors: A Blueprint for Targeting Protein–Protein Interactions" provides a comprehensive overview of the evolving landscape of drug discovery targeting protein-protein interactions (PPIs), which were long considered "undruggable" due to their large, flat surfaces. The authors review the conceptual and technical breakthroughs, including rational design principles and high-throughput screening that have made these interfaces accessible to therapeutic intervention.

The Arora Group
By using the Ras protein as a primary case study for a historically challenging target, the paper details how computational approaches like alanine scanning and the identification of "hot spots" allow for the dissection of protein surfaces. Furthermore, the review explores the design of macrocycles, miniproteins, and peptidomimetics that mimic natural binding epitopes, offering a strategic framework for creating potent inhibitors that can effectively modulate complex biological pathways.