Bacteria talk to each other. Through quorum sensing, QS, they release signaling molecules called autoinducers, monitor their concentration in the environment, and coordinate group behaviors once a critical threshold is reached. In streptococci, these signals take the form of competence-stimulating peptides, CSPs, that regulate a cascade of genes collectively known as the competence regulon. While this system primarily governs genetic competence, emerging research links it to other phenotypes, including hydrogen peroxide production, a potent weapon in the microbial arms race within the oral microbiome.
Streptococcus gordonii is an early colonizer of the oral cavity and generally contributes to oral health. Previous studies established that this species produces hydrogen peroxide via the enzyme pyruvate oxidase, SpxB, but the connection between this phenotype and the competence regulon remained unexplored. In a study published in ACS Infectious Diseases, researchers from the Tal-Gan Group at the University of Nevada at Reno, and The Bertucci Lab at Lafayette College, set out to map this relationship by isolating and verifying the native S. gordonii sp. firmicutes CSP, conducting transcriptomic analyses, and performing systematic structure-activity relationship, SAR, studies.
Group members from the two labs first confirmed the CSP as the 19-mer peptide with the sequence DIRHRINNSIWRDIFLKRK using high-resolution mass spectrometry and tandem MS/MS analysis. RNA sequencing revealed significant upregulation of competence-related genes following CSP exposure, including comABCDE regulon components and ComG operon genes involved in DNA uptake. A luciferase reporter system enabled precise quantification of ComD receptor activation, setting the stage for comprehensive SAR analysis. Alanine scanning, D-amino acid substitution, and truncation studies revealed that the N-terminus is essential for receptor binding and activation, while the C-terminus tolerates modification but not removal. Three analogs showed enhanced activity compared to the native peptide: CSP-R12A, CSP-L16A, and CSP-i6.
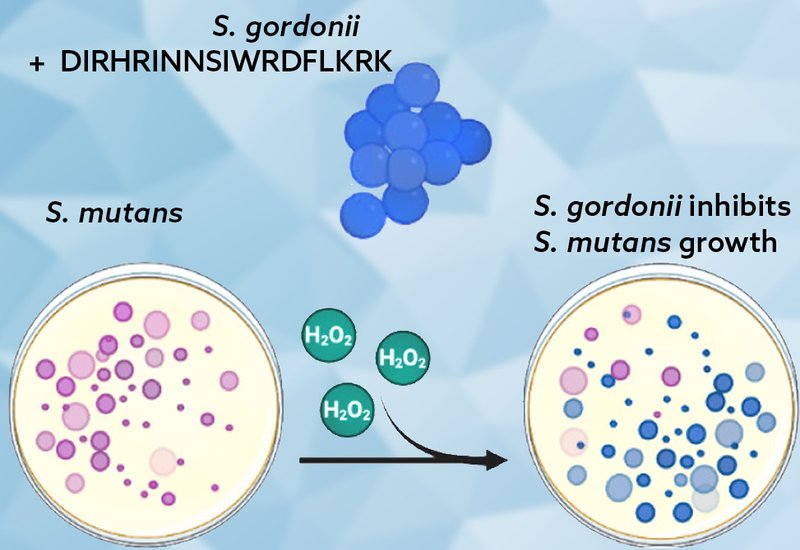
Phenotypic assays demonstrated that hydrogen peroxide production increased significantly with CSP concentration, and qPCR confirmed upregulation of spxB at 20 minutes post-CSP treatment. Interspecies competition assays showed that S. gordonii effectively inhibited growth of the pathogenic Streptococcus mutans, a major driver of dental caries. When catalase was added to degrade hydrogen peroxide, inhibition zones shrank dramatically, confirming the central role of peroxide in this antagonism.
These findings position S. gordonii as a potential biotherapeutic agent. By exploiting its QS-regulated hydrogen peroxide production, researchers may develop strategies to combat S. mutans infections and promote oral health through targeted manipulation of the oral microbiome.