Peptide drugs offer high target selectivity, strong efficacy, and low production costs, yet their clinical potential remains limited by rapid degradation in the bloodstream. Native glucagon-like peptide-1, for example, survives only minutes after intravenous administration. Researchers have extended the half-lives of peptide therapeutics by conjugating them to large circulating proteins such as serum albumin or immunoglobulin G antibodies. IgG antibodies persist in the blood for days thanks to recycling by the neonatal Fc receptor, and this strategy underpins blockbuster GLP-1 receptor agonists now used to treat type 2 diabetes and obesity. However, current IgG-drug conjugates require costly production and purification of exogenous, engineered antibodies, and these foreign proteins risk triggering unwanted immune responses.
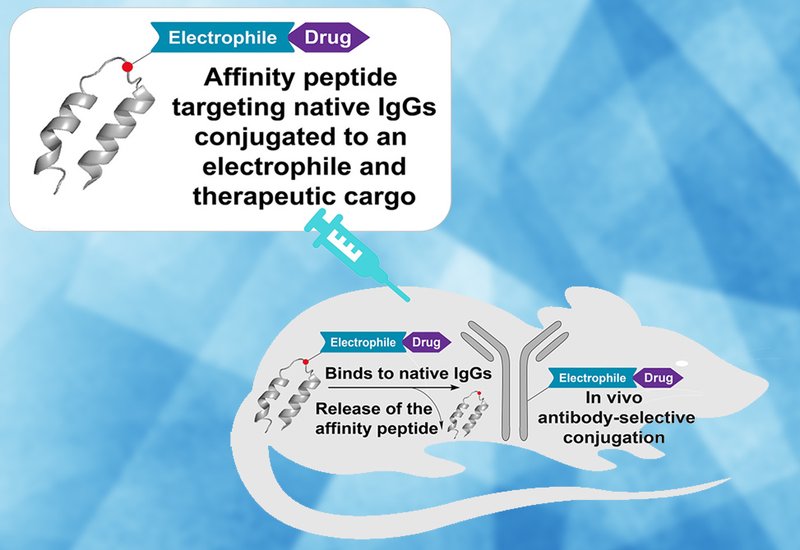
Researchers in the Pentelute Group at the Massachusetts Institute of Technology, collaborating with several other institutes and groups at MIT, published in Chem, have developed a bioconjugation platform that attaches therapeutic payloads directly to native IgG antibodies already circulating in the body. The team calls their approach "in vivo antibody-selective conjugation," and it eliminates the need for exogenous antibody engineering entirely.
The technology centers on a redesigned variant of Z33, a 33-amino-acid peptide derived from the minimal Z-domain of staphylococcal protein A that binds the Fc fragment of IgG1 with nanomolar affinity. The researchers substituted a key glutamate residue at position 20 with L-homocysteine to introduce a handle for site-specific conjugation of an electrophilic warhead. They then attached azide-functionalized electrophiles to this handle through palladium-mediated S-arylation using Pd-oxidative addition complexes. The resulting electrophilic affinity peptides, synthesized by automated fast-flow peptide synthesis, contain three modular elements: the Fc-binding domain, the reactive electrophile, and the drug payload. The team prepared and tested multiple Z33 variants with different substitution positions and electrophilic warheads to optimize selectivity and reaction efficiency. Once administered in vivo, the affinity peptide recognizes a specific lysine residue on the IgG Fc domain heavy chain, and a proximity-driven reaction transfers the payload covalently onto the antibody. The reaction proceeds at physiological pH and 37 °C without any catalyst and requires no prior chemical modification of the host antibodies.
To validate the platform, the team conjugated GLP-1 to circulating IgGs in two mouse models: wild-type lean Swiss mice and Lepob/Lepob mice with spontaneous obesity-related type 2 diabetes. A single dose of the GLP-1 conjugate produced sustained body weight loss and prolonged blood glucose control, confirming that the IgG-bound peptide retained its biological activity while benefiting from the extended circulation of its antibody carrier. Mass spectrometry confirmed selective modification of IgG heavy chains with minimal off-target labeling of other serum proteins.
The platform also proved versatile well beyond GLP-1. The researchers demonstrated successful in vivo conjugation of small molecules, additional peptide payloads, and radionuclides to circulating IgGs. Perhaps most notably, the team showed that two different drug cargoes could be loaded onto a single antibody molecule by targeting distinct lysine residues on the Fc domain with sequential reactions using different electrophilic warheads. Approximately 91% of heavy chains were successfully modified with two or more payloads while 86% of light chains remained unaffected, highlighting the precision of the approach and opening the door to combination therapies assembled directly in the bloodstream. By harnessing the body's own antibodies as drug carriers, this technology could dramatically reduce manufacturing costs while lowering immunogenicity risks, offering a compelling new paradigm for designing long-acting medicines across a broad range of diseases.